We are now offering a standalone version of the STARLIMS Scientific Data Management System (SDMS) to help clients achieve compliance with the data integrity expectations of the Food and Drug Administration (FDA) as well as other regulatory authorities.
Standalone SDMS can fill the gap for organizations that already invested in an existing LIMS that is lacking the Scientific Data Management System component. Organizations without a LIMS can utilize the standalone SDMS to better meet many of the requirements stated in 21 CFR Parts 211 and 212 for data integrity
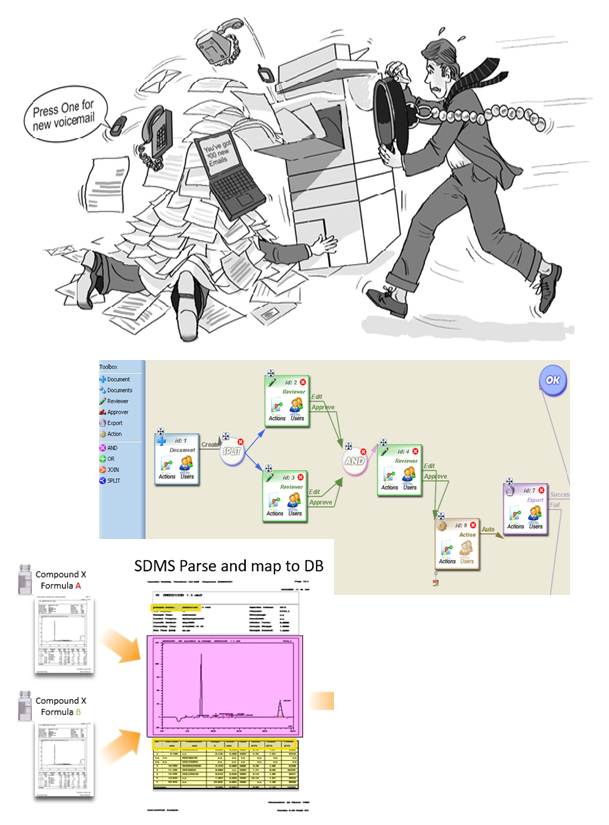
INSTRUMENT INTERFACING: Interface a variety of instruments with complete integrity and increased efficiency
TURNAROUND TIME: Captures data from instruments, increasing the productivity and quality of labs.
WORKFLOW: Create workflows for retrieval and approval of documents. Steps include actions like email, export to PDF, synchronize to LIMS, etc.
Centralized Data: Enterprise application lets users view/access data from different locations/sites with predefined permissions
DATA STORAGE & RETRIEVAL: For long term all storage, data and documents are indexed and easy to search and retrieve
COST SAVINGS: Instead of file servers and local computers, the SDMS database can store all instrument data.